Protein Chemical Labeling Using Highly Reactive Species
Protein chemical labeling, a technique to form covalent bonds between proteins and small molecules is in essential tool for the development of novel protein-based biomaterials and biopharmaceuticals, such as antibody-drug conjugates (ADC), the focus of intense research in recent years. To achieve protein chemical labeling, it is necessary to develop reactions that rapidly form covalent bonds with specific protein structures in water, at near-neutral pH, and at temperatures below 37°C. It has been a challenge to label tyrosine and histidine residues in protein structures, which are difficult to modify residue-selctively.
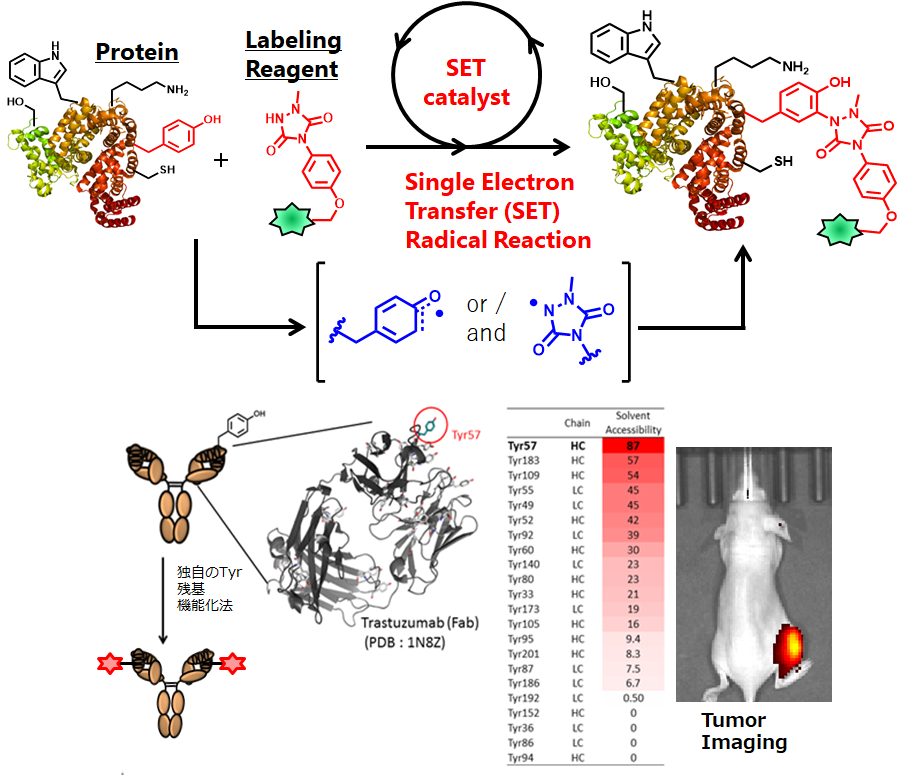
1.Tyrosine-Specific Modification
Functionalization of proteins by chemical modification requires mild reaction conditions that do not denature the protein, and organic chemical reactions that can be used in protein functionalization research have been limited to a few nucleophilic-electrophile reactions. As a result, only two amino acid residues (lysine and cysteine) out of the 20 naturally occurring amino acid residues can be modified with high reliability. In recent years, research targeting the other 18 amino acid residues has been actively conducted worldwide. We have developed methods for the modification of tyrosine residues using radical reactions. Through the chemical design of modifiers, and the logical design of tyrosine residue modification conditions based on the evaluation of redox potential, we have found efficient tyrosine-specific reactions that proceed under mild reaction conditions.
ACS Chem. Biol. 2015, 10, 2633
ChemBioChem. 2017, 18, 475
Bioconjate Chem. 2020, 31, 1417
Org. Biomol. Chem. 2020, 18, 3664
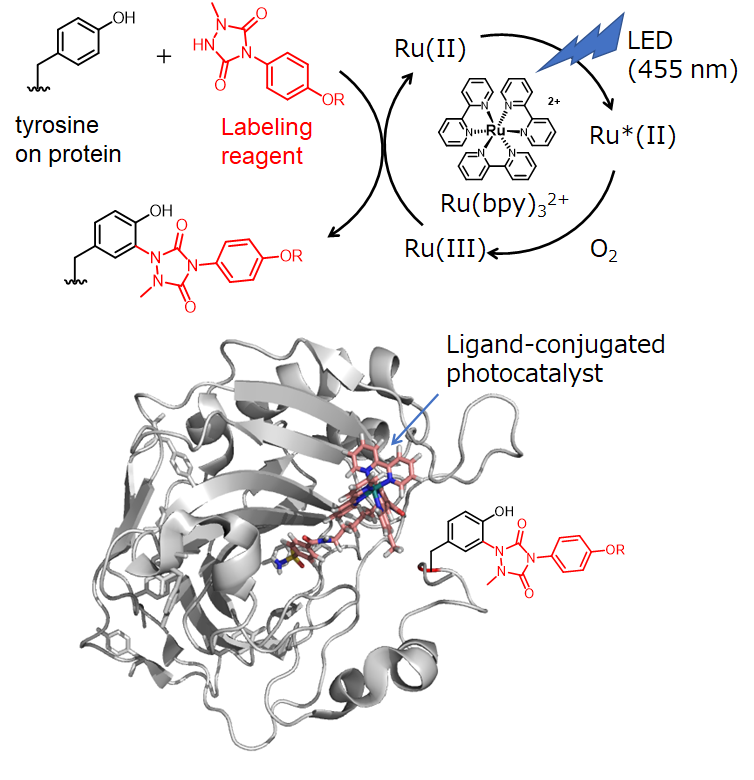
2.Photocatalyst-Proximity Protein Chemical Labeling
Light is an external stimulus that has little effect on animal cells and can be easily controlled. The method of controlling organic chemical reactions on proteins using light (photoaffinity labeling method) is widely used in the field of chemical biology research. However, there are problems such as the need for invasive UV light and low reaction efficiency. We have developed a photocatalytic proximity labeling method using the organometallic complex Ru(bpy)3 as a photoredox catalyst that can selectively induce radical protein labeling reactions in the surrounding environment using visible light stimuli such as 455 nm LED light. We have demonstrated a target-selective labeling method with superior efficiency compared to conventional photoaffinity labeling methods, and we have developed methods regulating the effective distance of radical reactions on a nanometer (nm) scale in close proximity to the catalyst
Angew. Chem. Int. Ed. 2013, 52, 8681
Bioconjugate Chem. 2015, 26, 250
Chem. Commun.2017, 53, 4838
Chem. Commun. 2018, 54, 5871
Chem. Commun.2019, 55, 13275
Chem. Commun. 2020, 56, 11641
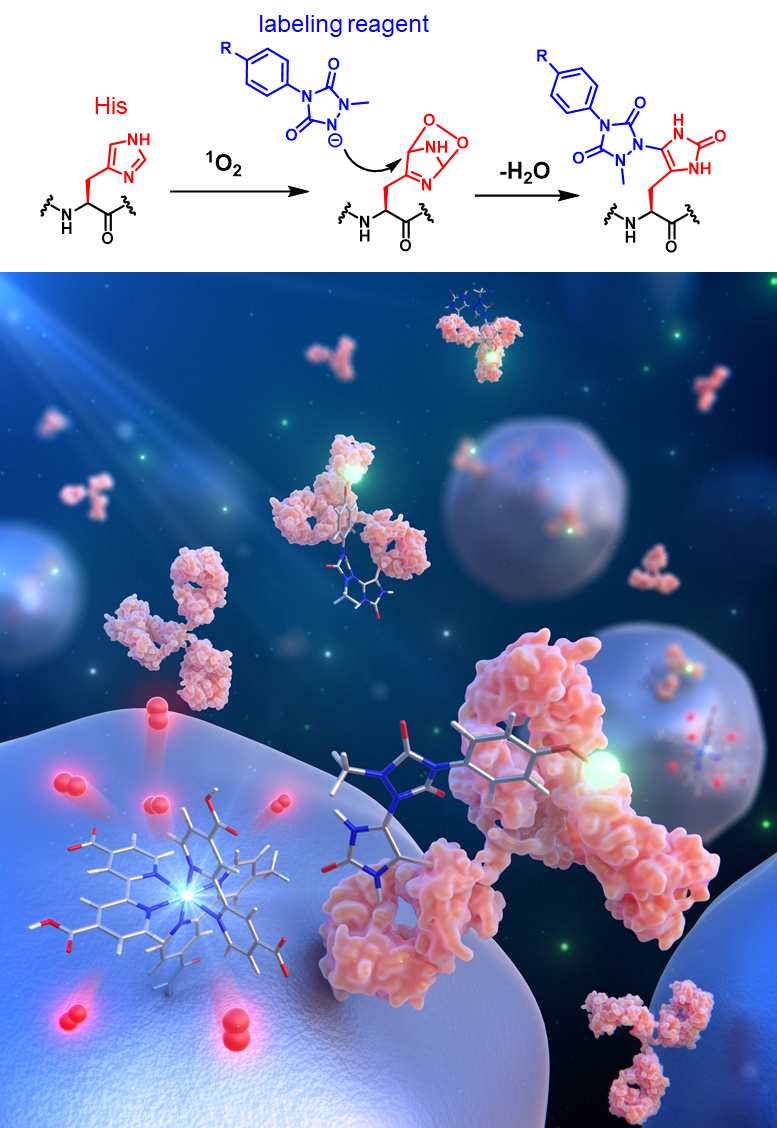
3.Proximity Histidine Labeling Using Single Oxygen
Singlet oxygen is a highly reactive reactive oxygen species produced from molecular oxygen upon light irradiation of a photocatalyst. Singlet oxygen is a short-lived (on the order of microseconds) and highly reactive species that is known to selectively oxidize proteins in the nanometer-scale proximity space around the catalyst. We have developed a method capturing electrophilic histidine intermediates with nucleophilic a labeling reagent. We succeeded in modifying histidine residues quickly (~minutes) and efficiently under physiological pH conditions. In addition, we assumed that site-selective histidine modification is possible by placing the photocatalyst in close proximity to specific sites on proteins. We succeeded in Fc-selective antibody chemical modification in the reaction field on magnetic beads functionalized with Fc-ligands and photocatalysts.
J. Am. Chem. Soc. 2021, 143, 7726
